Implementation of Whole Genome Sequencing as Screening in a Diverse Cohort of Healthy Infants
Robert C. Green, MD, MPH; Ingrid A. Holm, MD, MPH
Alan Beggs, PhD; Clement Bottino, MD, MPH; Kurt Christensen, PhD; Joy Dean, MD; Kelly East, MS; Bruce Gelb, MD; Carol R. Horowitz, MD, MPH; Bruce R. Korf, MD, PhD; Neil Lamb, PhD; Matt Lebo, PhD; Amy McGuire, JD, PhD; Stacey Pereira, PhD; Heidi Rehm, PhD; Jill O. Robinson, MA; Hadley S. Smith, PhD, MPSA; Hana Zouk, PhD
Background
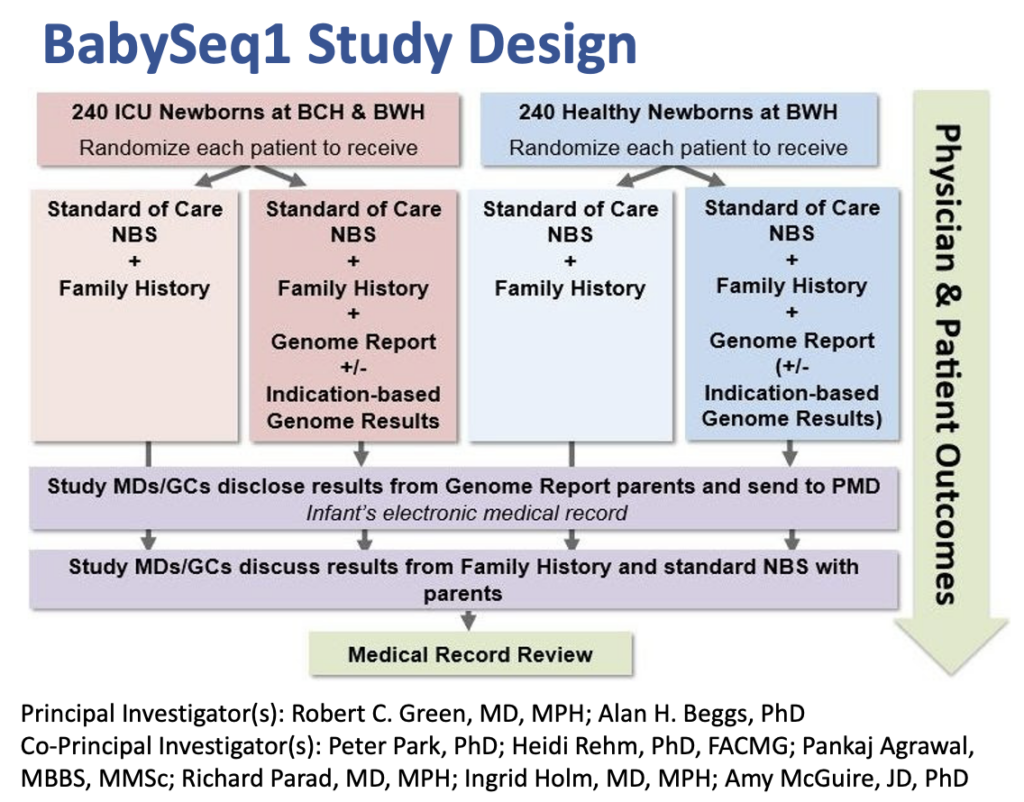
- The BabySeq Project: Phase I was a first-of-its-kind randomized clinical trial designed to measure the utility of using genomic sequencing in routine newborn care.
- The concept for this project is built off of the existing newborn screening program, an important public health initiative. All newborns born in the U.S. receive a heel stick blood test shortly after birth in order to screen for approximately 30 heritable, treatable conditions such as blood, endocrine, and metabolic disorders.
- Newborn genomic sequencing has the potential to supplement and expand existing newborn screening, by simultaneously screening for thousands of disorders that newborns could be at risk for developing during childhood.
- Earlier diagnosis of these conditions could in turn lead to specific screening, surveillance, and treatment options, allowing for more personalized and preventive healthcare.
Preliminary Data
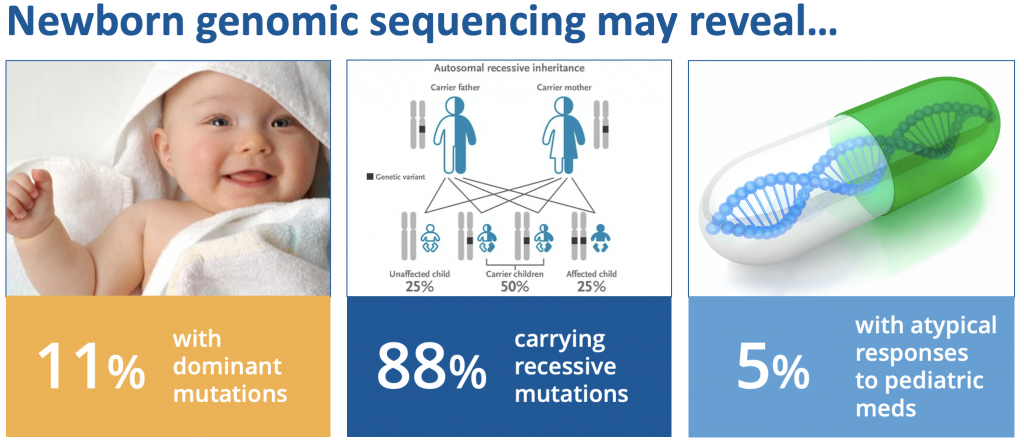
- The BabySeq project revealed unanticipated monogenic disease risks in 11% of newborn babies.
- Some infants already had signs or symptoms of the associated conditions, allowing for personalized management. You can hear one family’s story here.
- Other participants had a family history of related health problems, and this information helped their parents and relatives be more proactive about their health.
- 88% of newborns had at least one recessive carrier variant that could be relevant to their parents’ future reproductive planning, and 5% of babies had an atypical pharmacogenomic variant related to how they might process medications used in childhood.
- Parent surveys using validated measures showed no evidence that newborn genomic sequencing caused increased psychological distress (i.e. no increased risk of anxiety, depression, or family disruption compared to standard care) that persisted throughout the study, even if the baby had a disease risk identified.
- Analysis of the economic impacts of this type of testing is in progress. Preliminary models suggest that genomic screening could be cost-effective to prevent late-stage, expensive diseases.
Expansion and Implementation
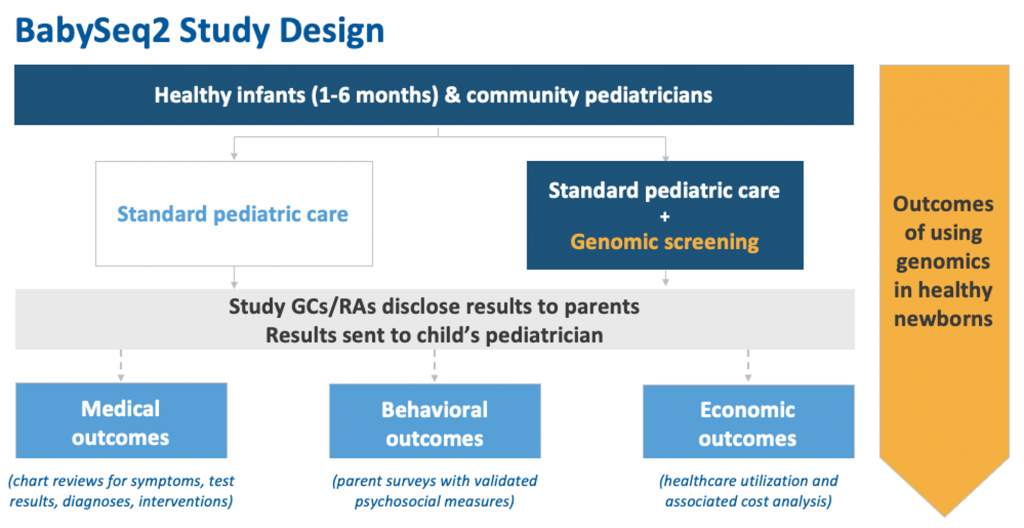
- The BabySeq2 Project is an implementation study that will expand and improve upon what we learned during the first iteration of the project under the direction of our co-Principal Investigators from Brigham and Women’s Hospital and Boston Children’s Hospital.
- We plan to enroll 500 new babies in Boston, MA; New York City, NY; and Birmingham, AL and prioritize inclusion of a diverse, nationally representative cohort of families.

- We will work with community members as partners throughout every step of the study to earn trust of potential participants, especially new parents who identify as Black/African American and/or Hispanic/Latinx.
- A key aspect of this community engagement strategy is our Stakeholder Board.
- We will also interview parents at each site (in English or Spanish) regarding their perspectives on genomic research.
- Each healthy baby will be randomized to receive genome sequencing or be in the control group (receive routine newborn care).
- Infants who receive genome sequencing will have their DNA analyzed for thousands of health risks that could affect their medical care in childhood.
- DNA may also be analyzed for a few highly actionable adult-onset conditions that could impact parents’ health.
- Results will be shared with the baby’s parents and pediatrician.
- We will also enroll pediatricians in the project to complete a brief training in genomics and follow-up with babies who have positive results. The study will collect data on how genomic information can impact healthcare throughout childhood.
For more detailed information about the BabySeq Project, including recruitment and eligibility details, study design and outcome measures, visit our clinical trial page:
For scientific papers and media coverage, or to donate to our BabySeq Project, visit the links below or email us at preventivegenomics@bwh.harvard.edu.








